Cerebrospinal fluid in Alzheimer’s diagnosis has emerged as one of the most significant advances in detecting and understanding this devastating neurodegenerative disease. For decades, a definitive Alzheimer’s diagnosis could only be confirmed through autopsy, leaving patients, families, and physicians navigating treatment decisions with considerable uncertainty. The analysis of cerebrospinal fluid (CSF)””the clear, colorless liquid that surrounds the brain and spinal cord””now offers a window into the molecular changes occurring in the brain years or even decades before memory loss becomes apparent. The importance of early and accurate Alzheimer’s diagnosis cannot be overstated. Approximately 6.7 million Americans currently live with Alzheimer’s disease, and that number is projected to reach 12.7 million by 2050.
Yet studies suggest that up to 30% of patients clinically diagnosed with Alzheimer’s based on symptoms alone may actually have a different form of dementia. This misdiagnosis problem affects treatment choices, clinical trial enrollment, family planning, and end-of-life decisions. CSF biomarkers address this gap by detecting the specific pathological proteins””amyloid beta and tau””that define Alzheimer’s disease at its biological core. By the end of this article, readers will understand what cerebrospinal fluid biomarkers measure, how the lumbar puncture procedure works, what results mean for patients and families, and how this diagnostic approach compares to other methods like PET imaging and emerging blood tests. Whether you are a caregiver researching options for a loved one, a patient considering testing, or a healthcare professional seeking to deepen your understanding, this comprehensive guide provides the scientific foundation and practical knowledge needed to navigate CSF-based Alzheimer’s diagnosis.
Table of Contents
- What Is Cerebrospinal Fluid and Why Does It Matter for Alzheimer’s Diagnosis?
- How CSF Biomarker Testing Detects Alzheimer’s Pathology
- The Lumbar Puncture Procedure for Cerebrospinal Fluid Collection
- Interpreting CSF Biomarker Results in Alzheimer’s Diagnosis
- Comparing CSF Testing to Other Alzheimer’s Diagnostic Methods
- The Future of Cerebrospinal Fluid Biomarkers in Dementia Research
- How to Prepare
- How to Apply This
- Expert Tips
- Conclusion
- Frequently Asked Questions
What Is Cerebrospinal Fluid and Why Does It Matter for Alzheimer’s Diagnosis?
Cerebrospinal fluid serves as both a protective cushion and a biochemical highway for the central nervous system. Produced primarily by the choroid plexus within the brain’s ventricles, approximately 500 milliliters of CSF circulates through the brain and spinal cord daily, with about 150 milliliters present at any given time. This fluid delivers nutrients, removes metabolic waste, and maintains the chemical environment neurons need to function. Because CSF directly bathes the brain tissue, it carries molecular signatures of whatever processes””normal or pathological””are occurring within the brain itself.
In Alzheimer’s disease, two abnormal protein accumulations define the condition: amyloid-plaques-in-living-brains/” title=”How PET Scans Detect Amyloid Plaques in Living Brains”>amyloid plaques formed by clumped amyloid beta peptides outside neurons, and neurofibrillary tangles made of hyperphosphorylated tau protein inside neurons. These changes begin fifteen to twenty years before cognitive symptoms appear. When amyloid beta aggregates into plaques in brain tissue, less of it flows freely into the cerebrospinal fluid, causing measurable decreases in CSF amyloid beta 42 (Aβ42) levels. Conversely, as neurons become damaged and die, tau protein leaks into the surrounding fluid, causing elevated CSF total tau (t-tau) and phosphorylated tau (p-tau) concentrations.
- **Amyloid beta 42 (Aβ42)**: This 42-amino-acid peptide is particularly prone to aggregation. In Alzheimer’s patients, CSF Aβ42 levels typically drop by 50% compared to healthy individuals, reflecting sequestration of the protein in brain plaques.
- **Total tau (t-tau)**: Elevated levels indicate neuronal injury and death. While not specific to Alzheimer’s””strokes and traumatic brain injuries also raise t-tau””it quantifies the extent of neurodegeneration.
- **Phosphorylated tau (p-tau)**: Specifically phosphorylated at threonine 181 or 217, p-tau is more specific to Alzheimer’s pathology and correlates strongly with tangle formation. Elevated p-tau helps distinguish Alzheimer’s from other dementias.
How CSF Biomarker Testing Detects Alzheimer’s Pathology
The diagnostic power of cerebrospinal fluid analysis lies in its ability to detect Alzheimer’s-specific changes with high sensitivity and specificity. Modern immunoassays can measure amyloid and tau proteins at picogram-per-milliliter concentrations, detecting abnormalities years before clinical symptoms manifest. Large-scale validation studies, including the Alzheimer’s Disease Neuroimaging Initiative (ADNI), have established that the combination of low Aβ42 and elevated p-tau achieves approximately 85-90% accuracy in identifying Alzheimer’s pathology when compared against amyloid PET imaging or autopsy confirmation.
The CSF Aβ42/Aβ40 ratio has emerged as an even more reliable marker than Aβ42 alone. Because Aβ40 production remains relatively stable regardless of plaque formation, dividing Aβ42 by Aβ40 controls for individual variations in overall amyloid production and CSF dynamics. Studies show this ratio improves concordance with amyloid PET scans to over 90%, reducing false positives and negatives that might occur when examining Aβ42 in isolation. Most clinical laboratories now report this ratio as standard practice.
- **Diagnostic sensitivity**: CSF biomarkers detect approximately 85-95% of individuals who truly have Alzheimer’s pathology, meaning few cases are missed.
- **Diagnostic specificity**: The tests correctly identify about 85-90% of individuals without Alzheimer’s, minimizing false positives that could cause unnecessary worry or inappropriate treatment.
- **Preclinical detection**: Abnormal CSF biomarkers can appear 15-20 years before dementia symptoms, identifying individuals in the earliest stages of the disease process when interventions may be most effective.
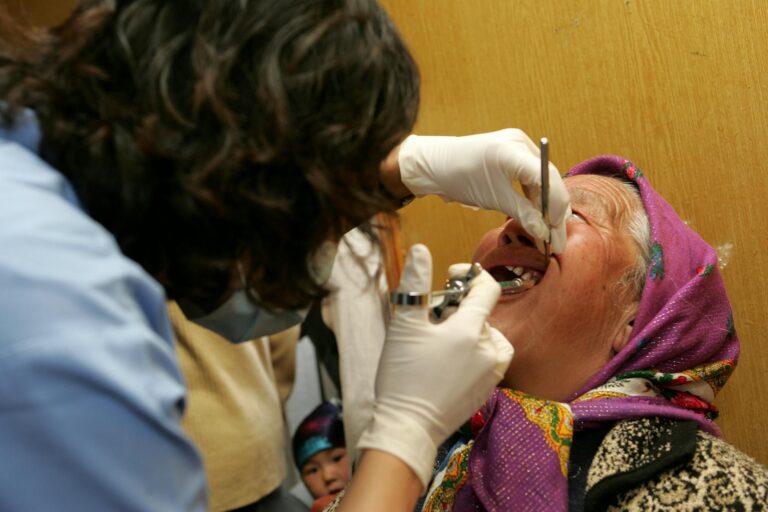
The Lumbar Puncture Procedure for Cerebrospinal Fluid Collection
Obtaining cerebrospinal fluid requires a lumbar puncture, commonly called a spinal tap. While the procedure’s reputation sometimes causes patient anxiety, modern techniques make it relatively straightforward with minimal complications. understanding what to expect can help patients and families approach testing with accurate expectations rather than unfounded fears.
During the procedure, the patient typically lies on their side in a fetal position or sits leaning forward, which opens the spaces between lumbar vertebrae. After cleaning the skin and applying local anesthetic, a thin needle is inserted between the L3-L4 or L4-L5 vertebrae””well below where the spinal cord ends””into the subarachnoid space where CSF circulates. The physician collects approximately 10-15 milliliters of fluid, which the body replaces within hours. The entire procedure usually takes 20-30 minutes, with the actual fluid collection requiring only a few minutes.
- **Post-procedure headache**: The most common side effect, occurring in 10-30% of patients, results from CSF leaking through the puncture site. Using smaller “atraumatic” needles reduces this risk to under 5%. Headaches typically resolve within days and respond to caffeine, hydration, and lying flat.
- **Infection and bleeding risks**: Serious complications are rare, occurring in fewer than 1 in 1,000 procedures. Sterile technique and avoiding patients on blood thinners (or managing anticoagulation appropriately) minimize these risks.
- **Contraindications**: Patients with brain masses causing increased intracranial pressure, bleeding disorders, or skin infections at the puncture site may not be candidates for lumbar puncture.
Interpreting CSF Biomarker Results in Alzheimer’s Diagnosis
Understanding cerebrospinal fluid test results requires recognizing that biomarkers exist on a continuum rather than as simple positive-negative categories. Laboratories report numerical values alongside reference ranges, and interpretation considers the entire biomarker profile rather than any single measurement. Clinicians integrate these results with cognitive testing, brain imaging, medical history, and physical examination to reach diagnostic conclusions.
A typical Alzheimer’s biomarker profile shows decreased Aβ42 (or decreased Aβ42/Aβ40 ratio), elevated p-tau, and elevated t-tau. The ATN classification system, adopted by the National Institute on Aging and Alzheimer’s Association, categorizes individuals based on Amyloid status (A), Tau pathology (T), and Neurodegeneration (N). Someone with abnormal amyloid and tau markers (A+T+) has biological Alzheimer’s disease regardless of current symptoms, while someone with abnormal amyloid alone (A+T-) is on the Alzheimer’s continuum but may not yet have significant tau pathology.
- **Concordance matters**: When CSF results align with clinical presentation””such as abnormal biomarkers in someone with progressive memory loss””diagnostic confidence is high. Discordant results, like normal biomarkers in someone with apparent dementia, prompt consideration of alternative diagnoses.
- **Borderline values**: Results near cutoff thresholds require careful interpretation. Repeat testing, additional imaging, or longitudinal monitoring may clarify uncertain cases.
- **Prognostic information**: Beyond diagnosis, biomarker levels provide prognostic information. Higher tau levels correlate with faster cognitive decline, while the degree of amyloid abnormality indicates plaque burden.
- **Treatment eligibility**: The new anti-amyloid monoclonal antibodies (lecanemab and donanemab) require confirmation of amyloid pathology before treatment. CSF testing or amyloid PET scanning satisfies this requirement.
Comparing CSF Testing to Other Alzheimer’s Diagnostic Methods
The landscape of Alzheimer’s biomarker testing has expanded considerably, giving patients and physicians multiple options for confirming pathology. Each method carries distinct advantages and limitations, and understanding these trade-offs helps in selecting the most appropriate approach for individual circumstances. Cost, availability, invasiveness, and diagnostic accuracy all factor into decision-making.
Amyloid PET imaging uses radioactive tracers that bind to amyloid plaques, allowing visualization of plaque distribution throughout the brain. This technology achieves similar diagnostic accuracy to CSF biomarkers and provides spatial information about where plaques have accumulated. However, PET scans cost significantly more””typically $3,000-6,000 compared to $300-800 for CSF analysis””and require specialized imaging centers that may not exist in rural areas. Tau PET imaging, a newer development, visualizes tangle distribution and correlates closely with cognitive symptoms but adds additional cost and radiation exposure.
- **Blood-based biomarkers**: Recent breakthroughs have enabled detection of p-tau217 and other Alzheimer’s markers in blood samples, offering a minimally invasive screening option. While not yet as accurate as CSF testing or PET imaging, blood tests are rapidly improving and may eventually become first-line screening tools, with abnormal results prompting confirmatory CSF or PET testing.
- **Cost considerations**: For patients without insurance coverage for PET imaging, CSF analysis provides equivalent diagnostic information at lower cost. Medicare covers CSF biomarker testing for appropriate clinical indications.
- **Radiation exposure**: Unlike PET imaging, CSF testing involves no radiation, which may be relevant for younger patients or those requiring serial monitoring.
- **Availability**: Lumbar puncture can be performed in most clinical settings, while PET imaging requires specialized equipment concentrated in academic medical centers and urban areas.

The Future of Cerebrospinal Fluid Biomarkers in Dementia Research
Beyond established markers like Aβ42 and p-tau, researchers are identifying new CSF biomarkers that could refine diagnosis, improve prognostication, and reveal novel treatment targets. Neurofilament light chain (NfL), a marker of axonal damage, helps track neurodegeneration across multiple conditions and may prove valuable for monitoring treatment response. Synaptic proteins like neurogranin indicate synaptic loss, potentially predicting cognitive decline before symptoms emerge.
Glial markers such as GFAP and YKL-40 reflect astrocyte and microglial activation, illuminating the neuroinflammatory component of Alzheimer’s pathology. The integration of CSF biomarkers with genetic testing, particularly for the APOE ε4 allele, enables increasingly sophisticated risk stratification. Someone with abnormal CSF biomarkers and two copies of APOE ε4 faces higher short-term conversion risk than someone with the same biomarker profile but no genetic risk alleles. As disease-modifying treatments become available, this precision medicine approach will help identify optimal treatment windows and appropriate candidates for intervention.
How to Prepare
- **Discuss medications with your physician**: Blood thinners like warfarin, aspirin, and newer anticoagulants increase bleeding risk during lumbar puncture. Your doctor may instruct you to pause these medications for several days before the procedure, weighing bleeding risk against the reason you take the medication. Never stop blood thinners without medical guidance.
- **Stay hydrated before the procedure**: Adequate hydration helps maintain CSF volume and may reduce post-procedure headache risk. Unless your doctor specifies fasting, drink plenty of water in the hours before your appointment. Avoid alcohol for at least 24 hours beforehand, as it contributes to dehydration.
- **Arrange transportation home**: While lumbar puncture does not require sedation, most facilities recommend having someone drive you home afterward. You may feel slightly lightheaded, and resting reduces headache risk. Plan to take it easy for the remainder of the day.
- **Wear comfortable, loose-fitting clothing**: You will need to bend your back during the procedure, either lying curled on your side or sitting hunched forward. Comfortable clothes make positioning easier. You may be given a hospital gown, but loose clothing simplifies preparation.
- **Prepare questions for your physician**: Write down questions about result timelines, what different outcomes might mean, and next steps based on findings. Understanding the bigger picture helps contextualize the procedure within your overall diagnostic journey.
How to Apply This
- **Schedule a comprehensive results discussion**: CSF biomarker results are not simply “positive” or “negative.” Request a dedicated appointment where your physician can explain what your specific values mean, how they fit with other testing, and what conclusions can be drawn. Bring a family member or friend to help process information.
- **Understand how results affect treatment options**: If biomarkers confirm Alzheimer’s pathology, discuss whether you might be a candidate for anti-amyloid therapies like lecanemab or donanemab. These medications require documented amyloid pathology and involve regular MRI monitoring for side effects. Biomarker confirmation opens doors to treatments unavailable without objective evidence.
- **Consider clinical trial participation**: Confirmed Alzheimer’s pathology””particularly in early stages””makes patients eligible for clinical trials testing promising new treatments. Organizations like the Alzheimer’s Association TrialMatch can connect patients with appropriate studies based on their biomarker status and disease stage.
- **Begin or adjust care planning**: Whether results confirm suspected Alzheimer’s or suggest a different diagnosis, use this information for practical planning. Discussions about future care preferences, legal documents, financial planning, and family communication are easier to have early in the disease course when the patient can fully participate.
Expert Tips
- **Request atraumatic needles**: Ask whether your facility uses pencil-point (atraumatic) needles rather than cutting (Quincke) needles. Research consistently shows atraumatic needles reduce post-lumbar-puncture headache rates from approximately 25% to under 5%, with no downside to their use.
- **Lie flat for an hour after the procedure**: While evidence is mixed on whether bed rest prevents headaches, many patients find that lying flat immediately after the procedure and avoiding strenuous activity for 24 hours reduces symptoms. This brief inconvenience is worth the potential benefit.
- **Understand that one normal result does not rule out future Alzheimer’s**: Biomarker abnormalities develop over time. A normal result in a 55-year-old with a family history does not guarantee permanent protection but rather indicates that detectable pathology has not yet developed. Repeat testing in several years may be appropriate for high-risk individuals.
- **Know that CSF results remain stable**: Unlike cognitive tests that fluctuate with daily factors like sleep and mood, CSF biomarker levels reflect accumulated pathology and remain relatively stable over months to years. A single sample provides reliable information without need for repeated testing to confirm findings.
- **Combine CSF results with clinical evaluation**: Biomarkers alone do not diagnose dementia””they confirm or exclude Alzheimer’s pathology. Someone with abnormal biomarkers but no symptoms has preclinical Alzheimer’s disease, not dementia. Comprehensive evaluation includes cognitive testing, functional assessment, and consideration of non-Alzheimer’s contributions to symptoms.
Conclusion
Cerebrospinal fluid biomarker testing represents a fundamental shift in how Alzheimer’s disease is diagnosed, moving from symptom-based clinical judgment to objective measurement of disease-defining pathology. The ability to detect amyloid and tau abnormalities years before cognitive decline transforms Alzheimer’s from a diagnosis of exclusion made late in the disease course to a precisely defined biological entity identifiable at its earliest stages. For patients and families navigating the uncertainty of cognitive changes, CSF analysis provides answers that clinical evaluation alone cannot deliver””confirming suspicions, ruling out feared diagnoses, or revealing unexpected findings that change the care trajectory entirely. The arrival of disease-modifying treatments makes accurate, early diagnosis more important than ever.
Anti-amyloid therapies offer greatest benefit when started early, before extensive neurodegeneration has occurred, but require confirmed amyloid pathology for appropriate use. CSF biomarkers provide this confirmation reliably and cost-effectively, complementing clinical assessment with molecular precision. As research continues to refine existing markers and discover new ones, the window into brain health that cerebrospinal fluid provides will only become clearer. Patients concerned about memory changes or at elevated genetic risk should discuss biomarker testing with their physicians to understand whether and when such testing makes sense for their individual situations.
Frequently Asked Questions
How long does it typically take to see results?
Results vary depending on individual circumstances, but most people begin to see meaningful progress within 4-8 weeks of consistent effort. Patience and persistence are key factors in achieving lasting outcomes.
Is this approach suitable for beginners?
Yes, this approach works well for beginners when implemented gradually. Starting with the fundamentals and building up over time leads to better long-term results than trying to do everything at once.
What are the most common mistakes to avoid?
The most common mistakes include rushing the process, skipping foundational steps, and failing to track progress. Taking a methodical approach and learning from both successes and setbacks leads to better outcomes.
How can I measure my progress effectively?
Set specific, measurable goals at the outset and track relevant metrics regularly. Keep a journal or log to document your journey, and periodically review your progress against your initial objectives.
When should I seek professional help?
Consider consulting a professional if you encounter persistent challenges, need specialized expertise, or want to accelerate your progress. Professional guidance can provide valuable insights and help you avoid costly mistakes.
What resources do you recommend for further learning?
Look for reputable sources in the field, including industry publications, expert blogs, and educational courses. Joining communities of practitioners can also provide valuable peer support and knowledge sharing.